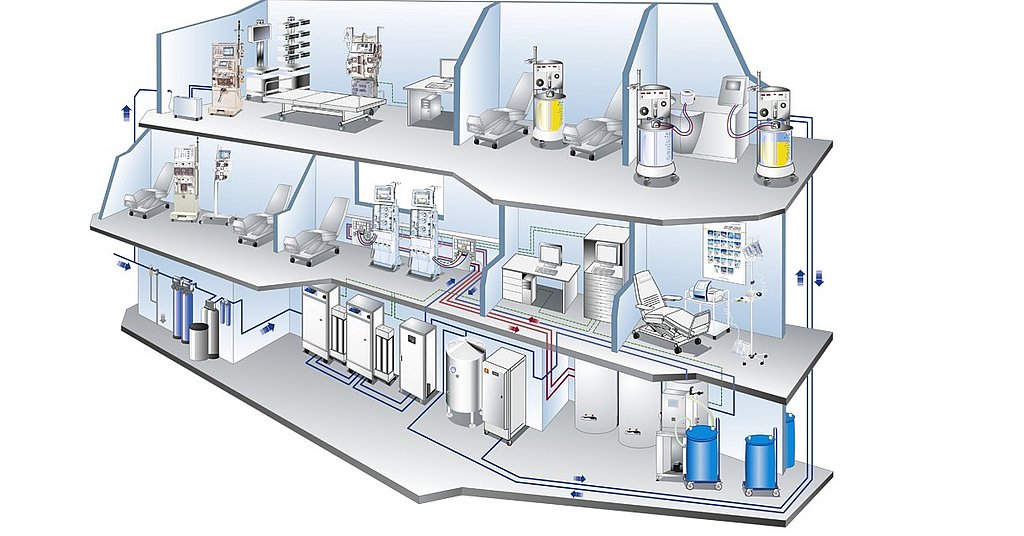
HighVolume HDF is Fresenius Medical Care.
Hygiene
Hygiene aspects in dialysis
| The spectrum of virucidal activity | |
|---|---|
| Test viruses to declare “limited spectrum of virucidal activity”1,2 |
|
| Test viruses to declare “virucidal”1,2 |
|
| Test virus to declare “virucidal” according to DIN EN 144763 |
|